Are Aβ Blood Tests Ready for Prime Time?
Quick Links
In the short space of two years, the erstwhile fantasy of a blood test for Alzheimer’s disease has become reality. Or so it seems. At the 2017 Alzheimer’s Association International Conference in London, Randall Bateman, Washington University, St. Louis, had wowed the audience by showing how an exquisitely sensitive mass spectroscopy assay of plasma detected a teeny drop in the Aβ42/40 ratio that correlated with positive PET scans for brain amyloid (Jul 2017 conference news). Scientists wanted to see this reproduced in other cohorts. At this year’s AAIC, held July 14-18 in Los Angeles, presentations on plasma Aβ measures abounded. Since last summer, as many as 11 different tests, both mass spec and immunoassays, have correlated plasma Aβ42/40 with CSF Aβ and amyloid in the brain. Is plasma Aβ ready for prime time? At AAIC, some researchers said yes, but others tempered their enthusiasm. Disagreement among mass spec and immunoassays emerged as a sticking point.
- Plasma Aβ appears as good as CSF or PET for diagnosis.
- Blood tests cut time, cost of screening for amyloidosis.
- But the assays don’t all agree.
Paul Aisen, University of Southern California, San Diego, was in the first camp. “The plasma assays are great,” he told Alzforum. “When they came out, I thought, ‘Everything is different now but we need to confirm.’ Well, it’s been confirmed.” Aisen is ready to use plasma assays in clinical trials, though he sees room for growth. “These assays are not in their final state and every improvement will make a difference,” he said.
Bateman is also gung ho. “I’m convinced this will work,” he told Alzforum. “In fact, it is working. We can take samples from different centers and run them in our mass spec assay and find total agreement,” he said, noting the assay is so robust that data from different sources can be pooled for analysis. Bateman co-founded C2N Diagnostics, St. Louis, which develops plasma assays, including for Aβ.
Others were more cautious, citing a lack of correlation among immunoassays, and between them and mass spec tests. This could reflect methodological issues, or different forms or pools of Aβ in plasma, noted Henrik Zetterberg, University of Gothenburg, Sweden. If those pools react differently with different antibodies, that might imply something important about the underlying biology, some researchers suggested. Oskar Hansson, Lund University, Sweden, also takes this view. “We need large—i.e., many hundreds of cases—head-to-head studies comparing different plasma Aβ assays in the same population against the same standard of truth,” he told Alzforum.
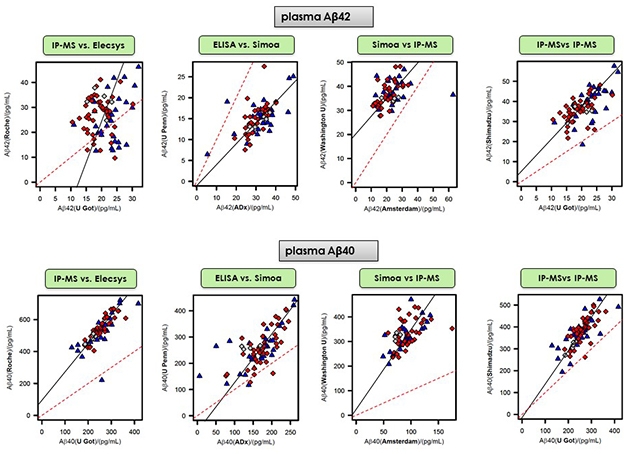
Scattershot? Some examples from a round robin comparing 11 different mass spec and immunoassays. It found generally weak correlations for measurements of Aβ42. This peptide appears to be particularly fickle, as the same tests posted slightly better correlations for measuring plasma Aβ40. Dashed red lines represent perfect correlations. [Courtesy of Henrik Zetterberg and Kaj Blennow, University of Gothenburg.]
Assays Galore
Since 2017, Akinori Nakamura and Colin Masters and colleagues published a mass spec method to measure the Aβ40/42 ratio; it is being developed by Shimadzu Corp. The team at UGothenburg optimized their own IP mass-spec assay, and the Spanish company Araclon has been working on such a mass-spec test service for some years already. Various immunoassays exist, as well, some currently in-house, some commercially available (see table below). All are being tested on various cohorts.
At AAIC, Bateman reported that his group has tested plasma of a further 158 people, members of the fourth in a line of cohorts being followed at the Knight Alzheimer’s Disease Research Center at WashU. As was the case in a previous sample set of 164 people, the plasma Aβ42/40 ratio once again tightly correlated with both the CSF Aβ42/40 ratio and with brain amyloid assessed by PET. All told, the WashU plasma test predicted amyloidosis with a specificity and a sensitivity of 76 and 88 percent, respectively, Bateman said. In statistical receiver operator curve analysis, this amounted to an area under the curve of 0.88. The AUC improved to 0.94 when the researchers factored in age and ApoE4 status, making this plasma Aβ test as good as CSF or PET for diagnosing amyloidosis. Led by Suzanne Schindler in Bateman’s lab, this work came out August 1 in Neurology (Schindler et al., 2019).
This mass spec test had predictive power. Of eight people whose amyloid PET scans were negative at baseline but positive on follow-up, seven had baseline plasma tests that were already positive, falling under the 0.1218 cutoff value for the Aβ42/40 ratio. In other words, in this small sample, people who were amyloid-negative by PET but amyloid-positive by plasma mass spec were 15 times more likely to test positive on PET later than were people who were plasma-negative at baseline.
What about other cohorts? In LA, Bateman reported results of testing samples from the Australian Imaging and Biomarkers Lifestyle study, the Swedish BioFINDER study, and the Alzheimer Disease Neuroimaging Initiative. The plasma test predicted PET status with AUCs of 0.87, 0.83, and 0.85 for AIBL, BioFINDER, and ADNI, respectively. Once again, adding ApoE4 into the equation raised these AUCs to 0.93, 0.90, and 0.87. Aβ42/40 cutoffs for amyloid positivity worked out to be 0.1235, 0.1234, and 0.1269 for AIBL, BioFINDER, and ADNI, respectively, which is close to the 0.1218 ratio calculated for the WashU cohort. Combined, for a total of 468 subjects, these three replication cohorts returned a cutoff of 0.1234.
For their part, immunoassays are posting similar results. At AAIC, Hansson reviewed his groups’ analysis of Roche Diagnostic’s Elecsys plasma immunoassay. This data appeared June 24 in JAMA Neurology (Jun 2019 news), and Alzforum covered it in depth. Briefly, this assay predicted amyloid positivity among 842 people in BioFINDER with about 80 percent accuracy, slightly less than the WashU test. In a validation cohort of 237 volunteers in a prospective study in Germany, the Elecsys immunoassay performed better, yielding an AUC of 0.86. In the Swedish cohort, adding in ApoE increased the AUC to 0.87; in the German cohort, genotyping was unavailable. In LA, Hansson also reported that among 335 cognitively normal people, the Elecsys plasma assay predicted who would get dementia over the next six years.
Head-to-Head Comparison: A Little Hairy
How do different plasma assays compare? Two AAIC presentations broached this question. Hansson showed preliminary head-to-head data for four such tests: Roche’s Elecsys immunoassay; EUROIMMUN’s ELISA; Shimadzu’s mass spec assay; and Quanterix’s antibody-based single-molecule array (Simoa). All but the Quanterix assay similarly predicted amyloid PET status among 199 cognitively normal people. The Elecsys, EUROIMMUN, and Shimadzu AUCs were 0.81, 0.76, and 0.82, respectively; the Simoa assay posted a 0.59. Adding APOE genotype improved these values to 0.86, 0.84, 0.87, and 0.79.
However, when you take a given set of samples, are the different assays measuring the same absolute amount of Aβ in it? Here it gets tricky. Zetterberg presented a comparison of 11 different plasma Aβ42/40 assays conducted at 11 different sites (see table below). Co-led by Kaj Blennow, also at UGothenburg, this round robin study was a project of the Global Biomarkers Standardization Consortium. The idea was to select plasma samples across a wide range of Aβ concentrations and send 0.25 milliliter aliquots to each participating lab, which tested these identical aliquots on their respective platforms. Unlike the work summarized in this story so far, this study did not evaluate any given test’s diagnostic accuracy against amyloid PET. Rather, it determined how the different tests correlated with each other when all were presented with the same amount of a given analyte—Aβ40 and Aβ42—in identical samples.

Who Participated. A round robin compared 11 different assays for plasma Aβ being conducted at 11 different sites. [Image courtesy of Henrik Zetterberg and Kaj Blennow, University of Gothenburg.]
So how did the tests match up? In short, not well, at least for Aβ42. Presenting slide after slide of pairwise correlations, Zetterberg showed results that scattered widely rather than crowding neatly around a line. For example, Elecsys values for Aβ42 matched poorly with MS data from UGothenburg, Shimadzu, and WashU, with the latter achieving the highest correlation of only 0.22 (see image below). Correlations of 0.27 and 0.46 between Elecsys and Simoa assays used by UPenn and UAmsterdam, respectively, were slightly better, but still weak. The best correlations, of around 0.6, emerged among the UGothenburg, Araclon, and Shimadzu mass spec assays. Calculating the Aβ42/40 ratio did not help, Zetterberg told Alzforum.
Tighter correlations, in the 0.6 to 0.7 range, emerged for the measurement of Aβ40, which is less sticky and occurs at 10 times higher concentration in plasma than does Aβ42. Still, Aβ42 is the key driver of amyloid pathology and the indicator of amyloidosis.

Head to Head. In this overview of correlations from the plasma Aβ round-robin study, green denotes tight correlations between any given two tests; red, weak correlations. [Courtesy of Henrik Zetterberg and Kaj Blennow, University of Gothenburg.]
Overall in this round robin, the mass-spec methods showed less variance among each other than the immunoassays did, Zetterberg said. This prompted debate about whether immunoassays or mass spec will be better. With the former, different antibodies might detect different pools of Aβ. The latter are harder to scale up, and dependent on at least one antibody for immunoprecipitation prior to mass spectrometry. Larger head-to-head comparisons with different clinical groups are next. “Before such studies have been done, I would not dare to say that all MS methods are superior to all immunoassays,” Hansson said.
Jonathan Schott, University College London, also found disagreement between MS and immunoassays. Working with Zetterberg and Blennow, Schott’s team compared the Quanterix Simoa to mass spec analyses run at UGothenburg. The plasma came from the 1946 British Birth Cohort, which is unique in that its participants, drawn from across the U.K., were all born within the same week in March of that year. They have been followed clinically since birth, and in the past few years, fluid and PET biomarkers were added.
For each volunteer, blood was collected at the same time of day, under the same fasting conditions, and treated and frozen the same way, Schott said. The scientists then measured Aβ40 and Aβ42 in plasma from 414 cognitively normal volunteers in the cohort.
For Aβ40, Simoa and mass spec data agreed fairly well, just like in the round robin. For the complete sample set, Quanterix returned a median of 288 pg/mL and mass spec 284 pg/mL, and comparing sample by sample, the two assays correlated with a coefficient of 0.44.
For Aβ42, the story was different. Mass spec measured higher values, giving a median of 28.4 pg/mL versus 19.5 pg/mL for Quanterix, and sample-by-sample correlation had a meager coefficient of 0.22. Regardless of whether a participant had a positive or negative amyloid PET scan, the MS measured more Aβ42 in the blood than the Quanterix test.
These differences are important. Schott found that the Aβ42/40 mass spec assay predicted PET positivity better than the Quanterix Simoa assay, with an AUC (uncorrected for age or ApoE status) of 0.82 over 0.61.
Why do the methods not agree? Zetterberg thinks it is not only the assays themselves, because these same assays did show tight correlations when used to measure Aβ42 in the CSF. Scientists at AAIC said the problem is likely the plasma. For one, plasma presents difficult matrix effects, meaning that the complex mixture of different solutes in the solution can cause spurious results. Diluting the plasma to get a more CSF-like matrix might help, but then the Aβ concentrations will be extremely low, requiring even more sensitive assays, Zetterberg said. It could also be that some assays are particularly sensitive to details of sample handling, which vary across centers. The immunoassays may be measuring different pools of Aβ; and at 10 to 40 picograms per milliliter, the Aβ42 concentration in plasma is always hovering dangerously close to the limit of detection.
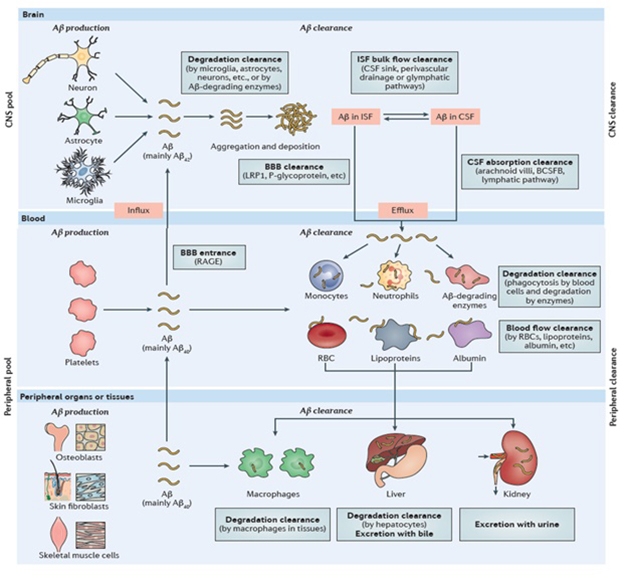
Bloody Complicated. Plasma Aβ comes from platelets, the brain, and peripheral organs. Systemic conditions influence plasma Aβ concentration. [Courtesy of Yan-Jiang Wang, Nature 2017.]
Does it matter how well assays correlate so long as they diagnose amyloidosis? Scientists seemed divided on this point. Blennow sees trouble down the road. “When different labs use different assays on identical aliquots that contain a given amount of Aβ42, and they measure quite different amounts—that is a problem,” he said (see Blennow Q&A). Colin Masters, University of Melbourne, Australia, echoed the sentiment, as did Ralph Martins from Edith Cowan University, Perth, Australia. They said that because the difference in plasma Aβ42 between controls and amyloid-positive people is so small to begin with, any biological phenomena that influence the blood Aβ concentration could tip the measurement. These phenomena include high blood pressure, diabetes, and hypoxia, and lifestyle factors, such as exercise.
Martins investigated the effect of physical activity on plasma Aβ42, and came up with a surprising result. Although evidence is strong that physical activity can protect the brain from dementia, among all healthy controls in AIBL, those who recorded high physical activity over the preceding seven days had less Aβ42 in their plasma than those who had reported low or medium activity. Less plasma Aβ42 could be taken to mean these people had more amyloid in the brain than people who exercised less. “The trend is exactly the opposite of what you might expect,” said Martins.
The trend held in healthy controls who tested negative for brain amyloid by PET, but in people who were amyloid-positive, physical activity seemed to have no effect on plasma Aβ42. Martins called the results perplexing, and surmised that exercise might promote Aβ degradation. “The take-home message is that lifestyle factors, particularly exercise, can influence plasma Aβ levels and need to be controlled for,” said Martins.
Those concerns prompted a study at WashU, where 1,000 people with common diseases such as hypertension, cancer, and diabetes are being enrolled for monitoring, periodic blood draws, and an amyloid PET scan in an effort to learn how various health conditions affect their blood Aβ42/40 ratios and its ability to predict their brain amyloidosis.
Meanwhile, companies are pushing to commercialize plasma assays. C2N and Shimadzu are already selling mass-spec tests to a few groups, and the other companies are not far behind. The stakes are high. Plasma assays will usher in a new era of diagnostics and prognostics in the AD field. Scientists at AAIC were giddy over the prospects of screening more effectively and cheaply for clinical trials. They envision running shorter trials with shifts in plasma Aβ as readouts, or of going back to thousands of blood samples stored from previous trials to tease out biological effects that may have been missed. Even basic questions such as Alzheimer’s incidence in developing countries will be easier to answer with a reliable blood test.
Bateman and Hansson both stressed the savings offered by plasma tests. Bateman calculated that in a trial such as A4, which used amyloid PET as an inclusion criteria, a plasma test could cut screening time from three years to six months and slash costs 10-fold. Hansson estimated that a plasma Aβ test could halve the number of PET scans needed to enroll 1,000 people, saving about $4 million. For his part, Schott based his estimate on a price tag of $3,000 for a PET scan and an assumed $400 for a blood test, calculating a saving of about $3.5 million for a trial of 500 participants.
That price assumption may prove optimistic. At AAIC, companies were marketing their plasma tests to customers such as pharma and large academic groups who are planning treatment trials and need to bring down screening costs. The companies are feeling out what they will be able to charge, but when asked about price, they were circumspect. When pressed, Shimadzu representatives allowed that they were currently selling their service in Japan at a list price of about $1,000 per test and an actual negotiated rate of between $500 and $900. Shimadzu intends to start selling for research use in the U.S. this fall. Its representatives told Alzforum that the pricing structure was not set yet, but would be somewhat lower than in Japan.
Like Shimadzu and Araclon, C2N sells its test as a service, where plasma gets shipped to its central lab and analyzed on the company’s mass-spec machines. C2N representatives told Alzforum that the current price for pharma and research use comes to roughly $700 per sample. Like Shimadzu’s, it is negotiated. C2N’s price varies depending on how many tests are being purchased, and whether the customer shares back data that C2N could use in its application for FDA approval. C2N has FDA breakthrough validation for its mass spec plasma Aβ assay, and is preparing an approval submission.
“This is our coming-out party,” Joel Braunstein of C2N told Alzforum during AAIC. “We are getting ready to stand behind our product. We have 30 meetings here to feel out what price pharma and academic groups find acceptable. Everyone is asking us what it will cost! We are envisioning below $1,000 per analyte.” For a Q&A on developing robust blood tests in Alzheimer's, see part 10 of this series. —Tom Fagan and Gabrielle Strobel
References
News Citations
- Finally, a Blood Test for Alzheimer’s?
- Drawing Closer: Alzheimer’s Blood Test for Primary Care
- Why Bother With Round Robins on Blood Tests? Q&A with Kaj Blennow
Paper Citations
- Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, Holtzman DM, Morris JC, Benzinger TL, Xiong C, Fagan AM, Bateman RJ. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019 Oct 22;93(17):e1647-e1659. Epub 2019 Aug 1 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, Holtzman DM, Morris JC, Benzinger TL, Xiong C, Fagan AM, Bateman RJ. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019 Oct 22;93(17):e1647-e1659. Epub 2019 Aug 1 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.